Explain How Atoms of Different Elements Are Different
Difference between Elements and Atoms. Maybe they can absorb a certain amount of energy or twice.
Chemistry I Atoms And Molecules
If false explain why it is false.
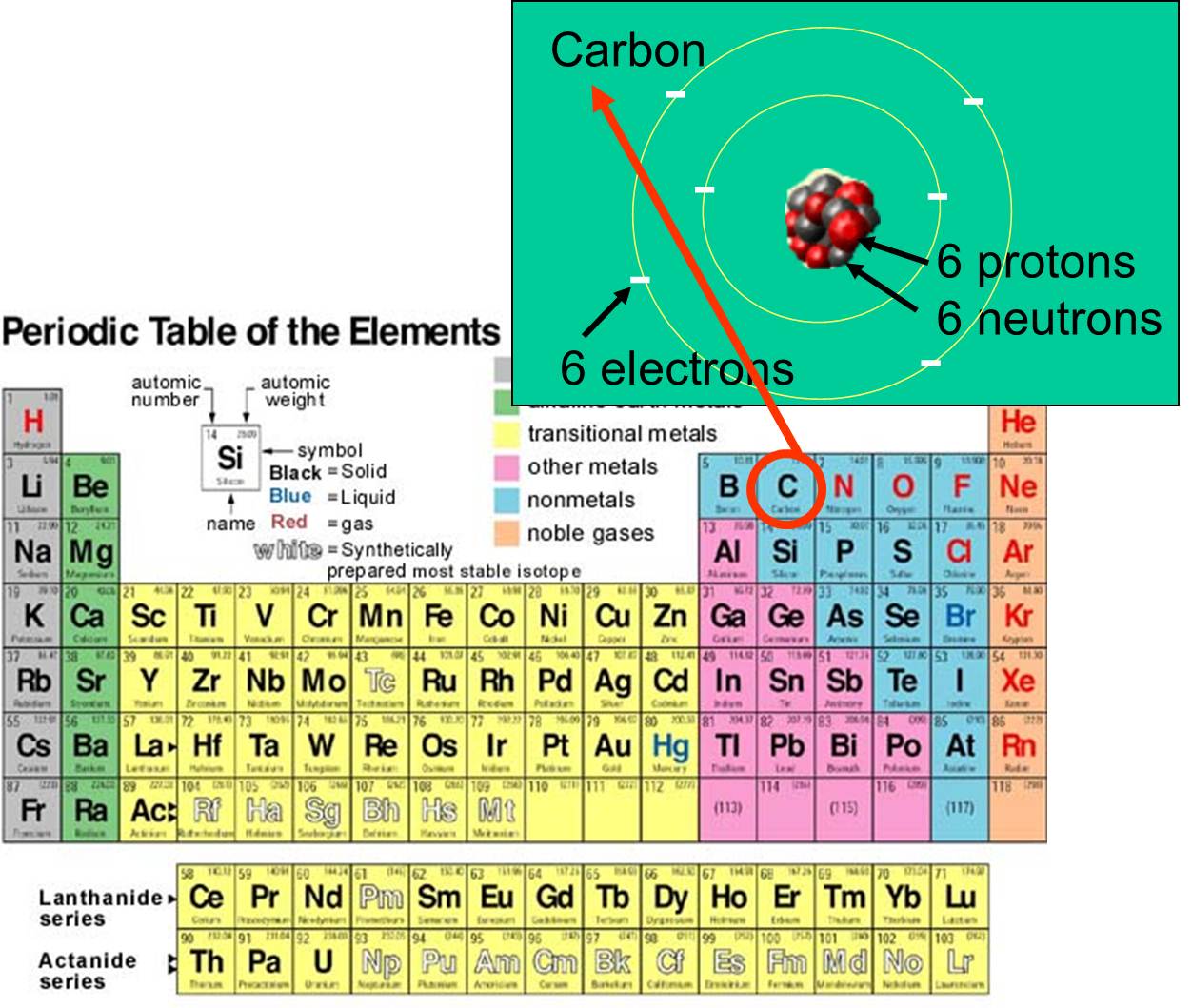
. This is because the atomic number is based upon the number of protons which is shown in pink. What makes one element different from another is the number of protons in the nucleus of its atoms. For example lithium atoms are larger than beryllium atoms and.
The emission lines correspond to the differences between various. Different colours of light have different energies. Explain how two atoms of oxygen can be different 1 See answer insightdomains is waiting for your help.
This problem has been solved. An element is the simplest form of a. An atom is the smallest particle.
It is important because without the neutrons the protons would repel each other. Lets compare two elements to answer this question. The atoms of different elements have different masses.
38 Votes Each elements emission spectrum is distinct because each element has a different set of electron energy levels. The packing is determined by the shape of the packed unit it looks for minimum energy configurations and by the desirability of interactions in different directions between the units. In what way are atoms of oxygen most different from and atoms of nitrogen.
The subatomic particle that makes atoms of different elements different from each other is the proton. 465 2279 Views. Add your answer and earn points.
The emission lines correspond to the differences between various pairs of the many energy levels. See the answer See the answer done loading. For example sodium atoms are larger than lithium atoms and potassium atoms are larger than sodium atoms.
To make a hydrogen atom you need one electron and one proton. Atomic size decreases with increasing atomic number across a period. The only orbitals available to fill are larger in size so that an electron attempted to be placed in one of these sees an attraction to the nucleus and a corresponding matching repulsion by all the electrons in the lower-energy compact orbitals.
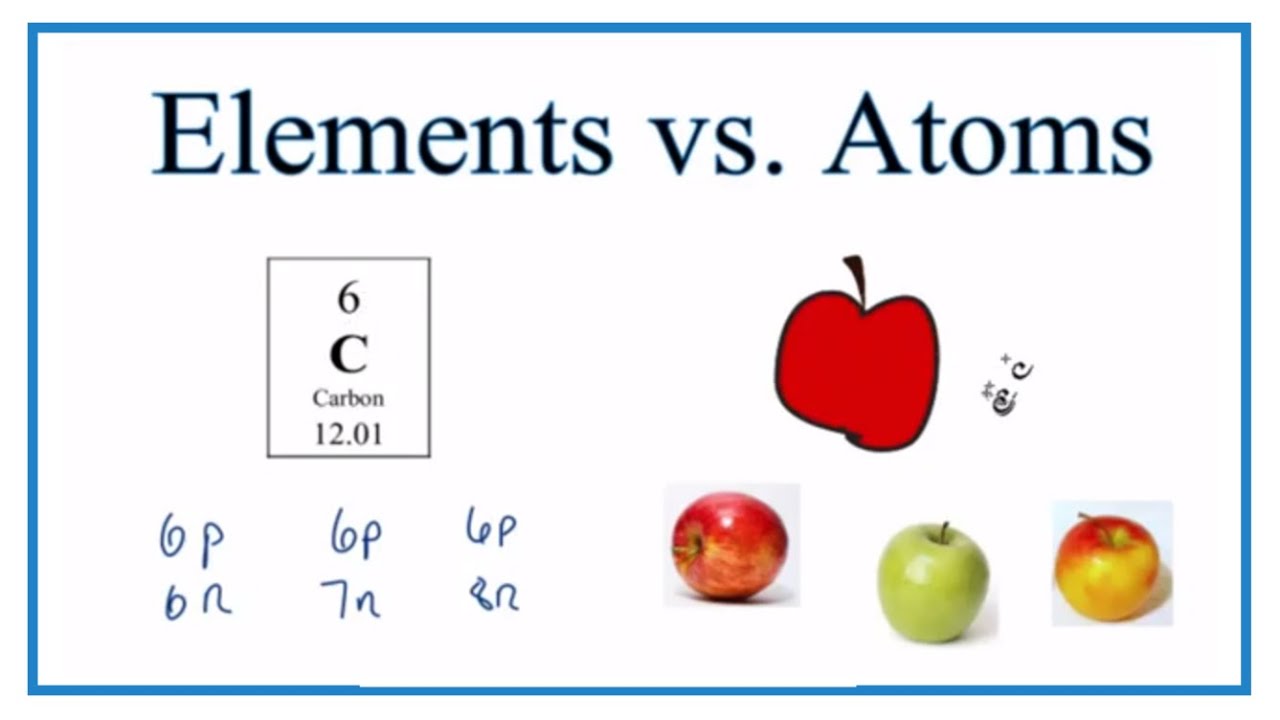
Only if the number of protons and consequently the electrons is different the atoms are of two different elements. 2 Explain how atoms of different isotopes of the same element differ from one another eg Carbon 12 vs Carbon 14. The bluer the light and light comes in blobs called photons the more energy the photon has.
A TOMIC M ASS A ND M OLECULAR M ASS Atomic mass unit It is. Each element is made up of one or more atoms. The lines photons are emitted as electrons fall from higher energy orbitals to lower energies.
Beside above why do different elements produce different spectra. The elements in the last family the column with He Ne Ar Kr Xe identify atoms with all the lower energy stabilizing orbitals completely filled. The electrons inside atoms can only have certain energies so they have what are called energy levels.
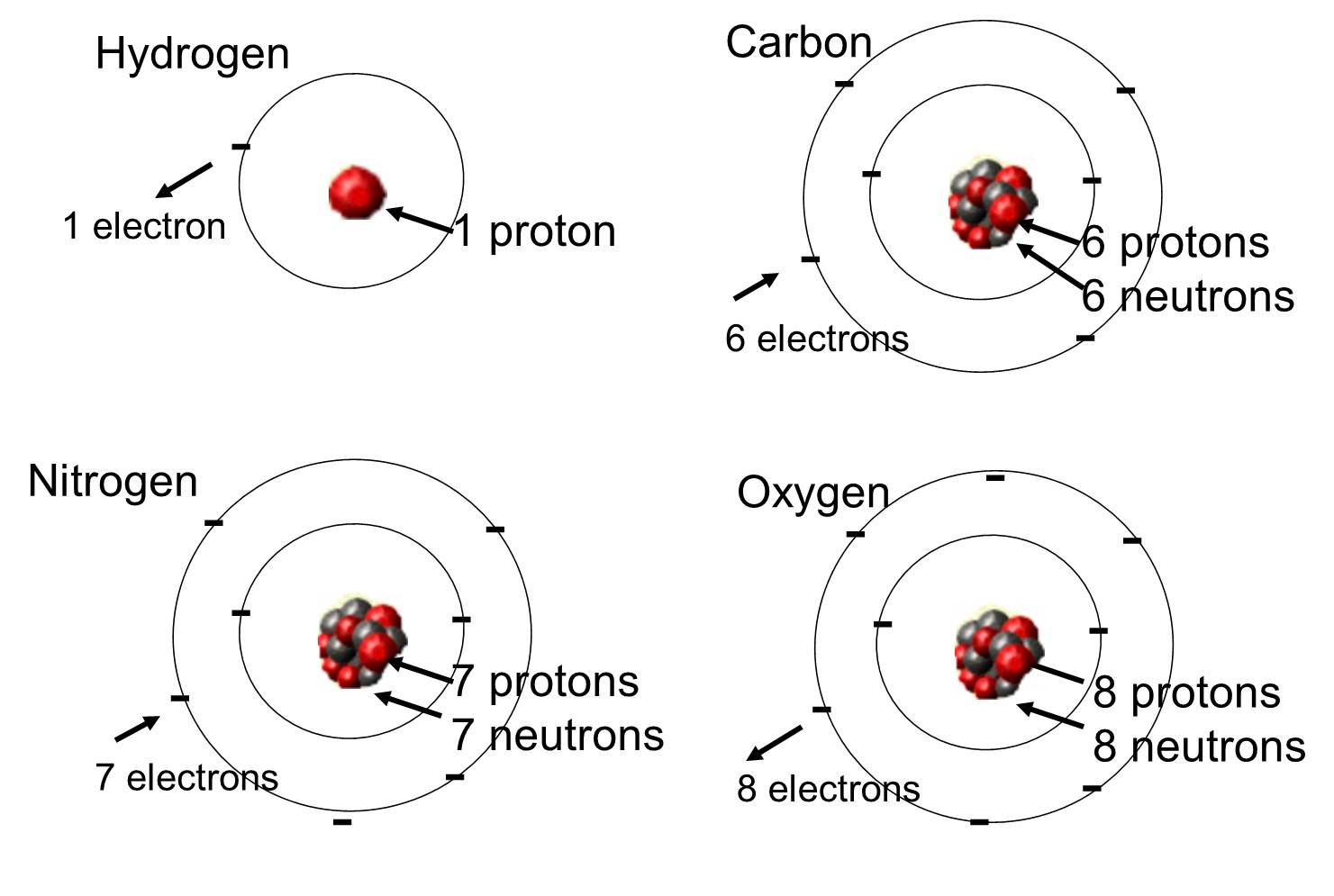
1Explain how atoms of different elements differ from one another use Carbon vs Nitrogen. It did not make any difference between ultimate particle of an element that takes part in reaction atoms and ultimate particle that has independent existence molecules. Explain how atoms of different isotopes of the same element differ from one another.
What makes atoms different is the number of protons neutrons and electrons. It will always have six protons if it does not have six pro tones. Thamimspartan thamimspartan Two atoms of an oxygen atom can be different if they are oxygen isotopes.
They have way different masses. Carbon has six protons. The biggest effect is actually what colour something absorbs.
This is given as the atomic number of the element on the periodic table. For mono-atomic elements basically packing. Yes it is very unique.
In a compound each element is represented by a letter and how many atoms each element has is represented by the subscript number if there is no number then there is 1 atom of that element. Of electrons protons and neutrons. Step 1 of 3.
It failed to explain how atoms of different elements differ from each other ie did not tell anything about structure of the atom. Those colors are a result of electrons in the atoms being excited up into higher energy levels by the heat of the flame. Atoms of different elements have different no.
A Atoms of different elements are different one another because of their different composition of protons electrons and neutrons. Explain how atoms of different elements differ from one anotherb. Answer 1 of 2.
Lets compare Carbon 12 and carbon 13 to explain why they have the same atomic number but different mass values. Due to different number of these sub-atomic particles different elements have different physical and chemical properties and reactivity. Each elements emission spectrum is distinct because each element has a different set of electron energy levels.
Determine whether the statement is true or false. You need 4 neutrons to make a beryllium atom.
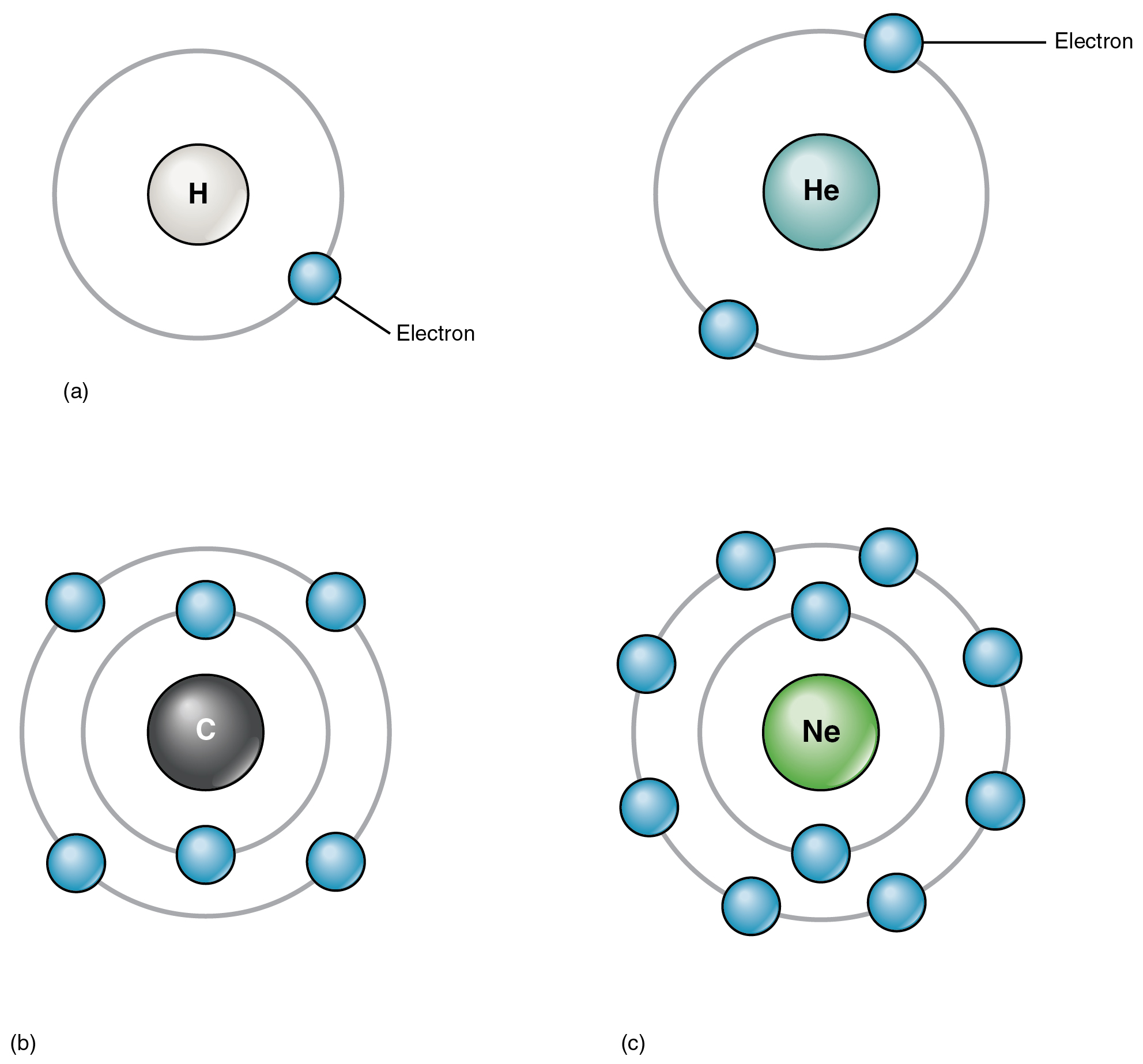
Elements And Atoms The Building Blocks Of Matter Anatomy And Physiology
Chemistry I Atoms And Molecules
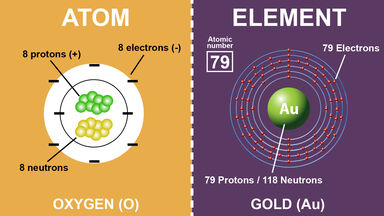
Difference Between Atoms And Elements With Examples
Understanding The Relationships Between Elements Molecules Compounds Video Lesson Transcript Study Com

What Is Atom How Does It Exist And It S Symbols Teachoo

Elements Atoms Molecules Ions Ionic And Molecular Compounds Cations Vs Anions Chemistry Youtube

Atoms And Elements Biology For Majors I
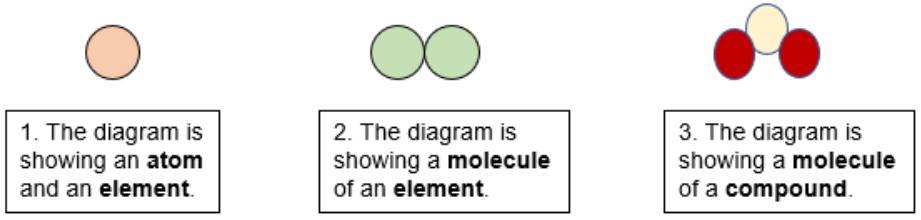
Understanding Atoms Elements And Compounds Lesson And Worksheets

Elements Atoms Molecules Ions Ionic And Molecular Compounds Cations Vs Anions Chemistry Youtube

Terminology Visual Explanation Between Molecule Vs Compound Vs Element Vs Atom Vs Substance Chemistry Stack Exchange
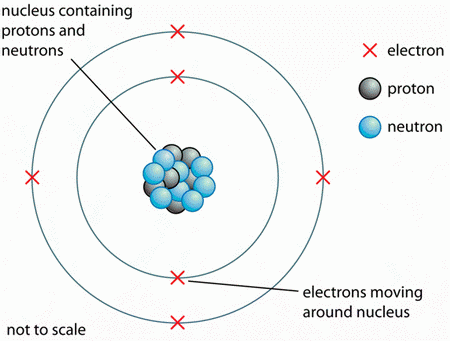
What Do All Atoms Of All Elements Have In Common Socratic

Compounds Facts Science Trek Idaho Public Television
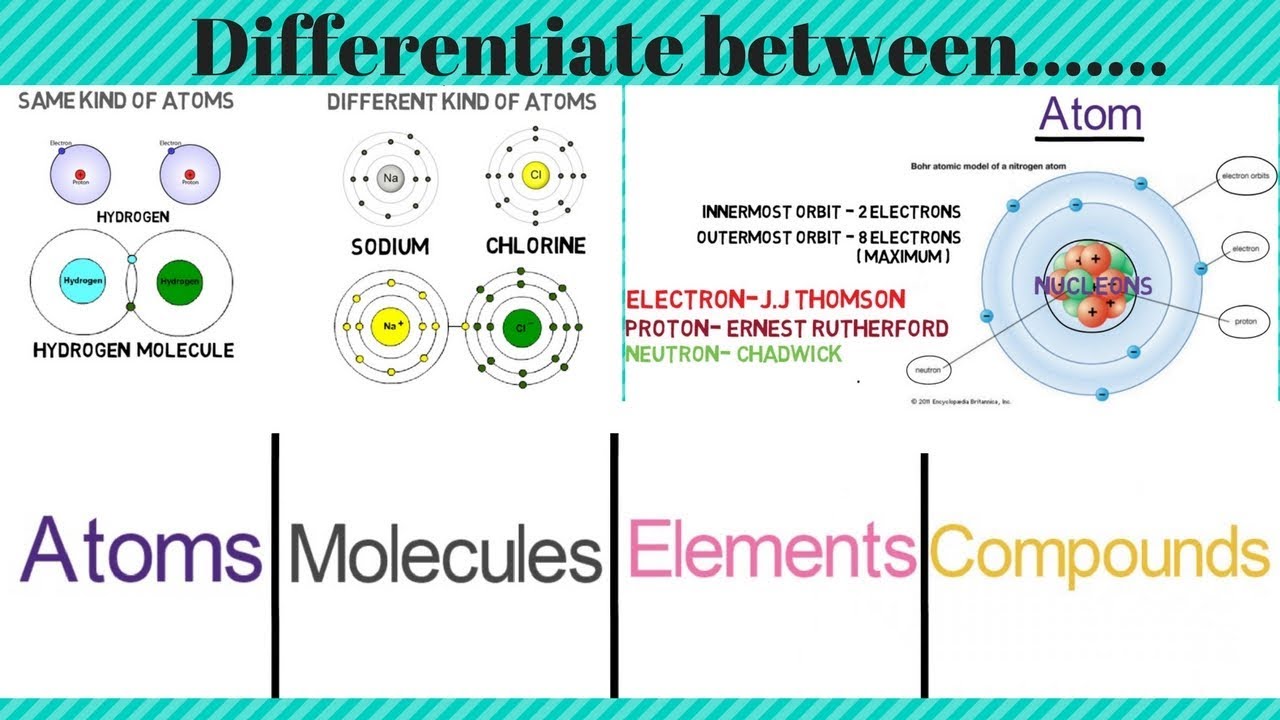
What Is The Difference Between Atom Molecule And Compound Lisbdnet Com
Atoms Isotopes Ions And Molecules Boundless Biology
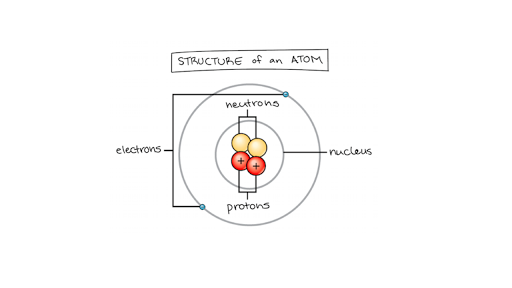
Matter Elements And Atoms Chemistry Of Life Article Khan Academy
Comments
Post a Comment